Impressions: hair identification
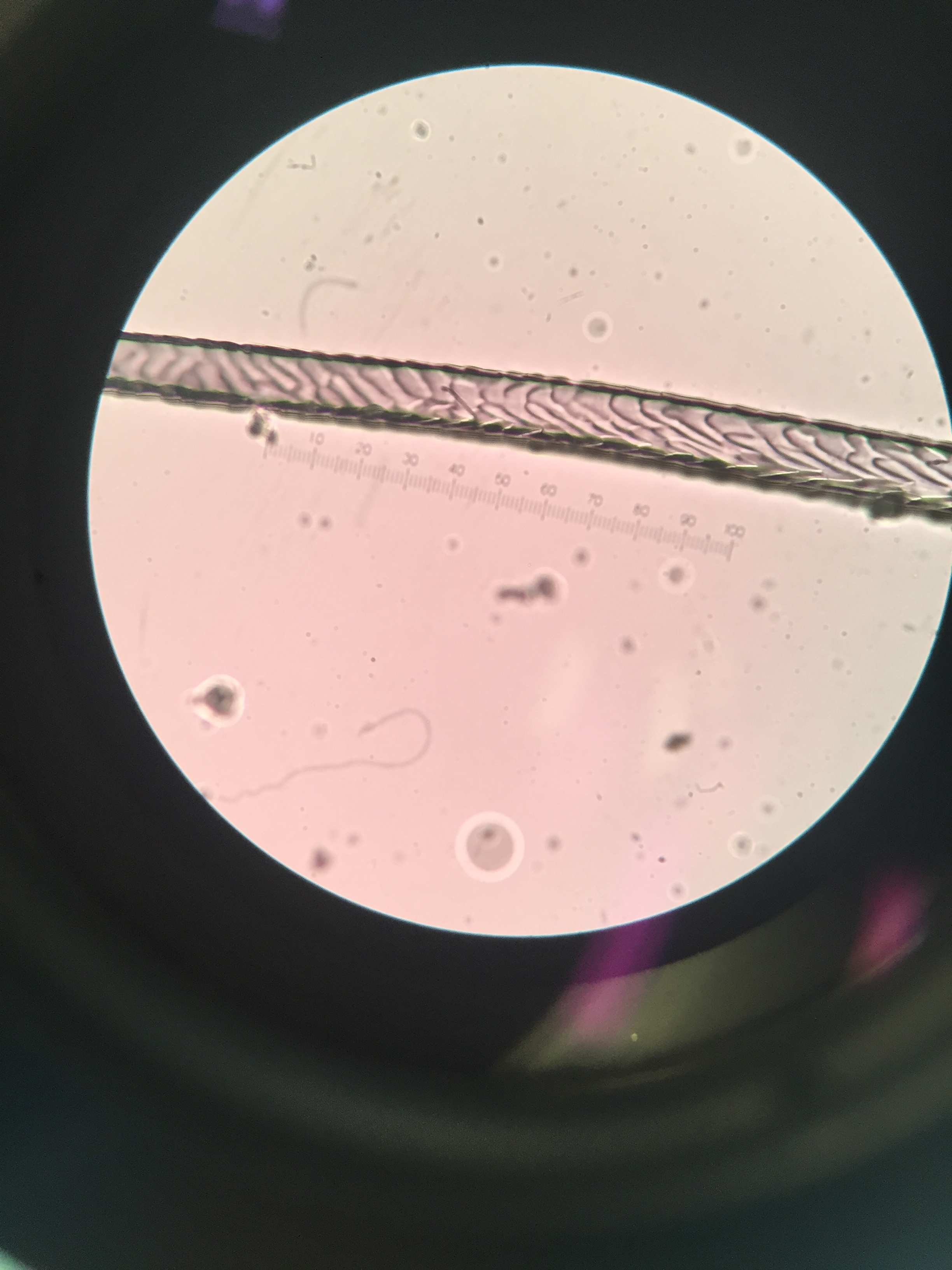
Another milestone reached: all the identifications are done! Of course, there are still some elusive kills and scat which I’ll never be 100% sure on, but not from lack of trying. The last step for identifying species once all other methods had failed was looking at hair cuticle scale patterns. This requires making an impression of the outside of the hair. In the old (and non-vegetarian) days, people used gelatine, but I just used clear nail polish, which works just as well. You place the hair into some (slightly dried) polish and, once dried, pull it off and examine the impression left behind under a microscope. The process also requires subsampling the hair from the scat beforehand (I used a numbered grid and a random number generator), and washing the hair in solvents to clean them of natural oils and dirt. This may all sound easy, but a lot of it requires dealing with individual hairs. I can tell you there is a particular kind of agony in trying to place a single, minuscule mouse hair into wet-but-not-too-wet nail polish, let alone trying to pull it out again.
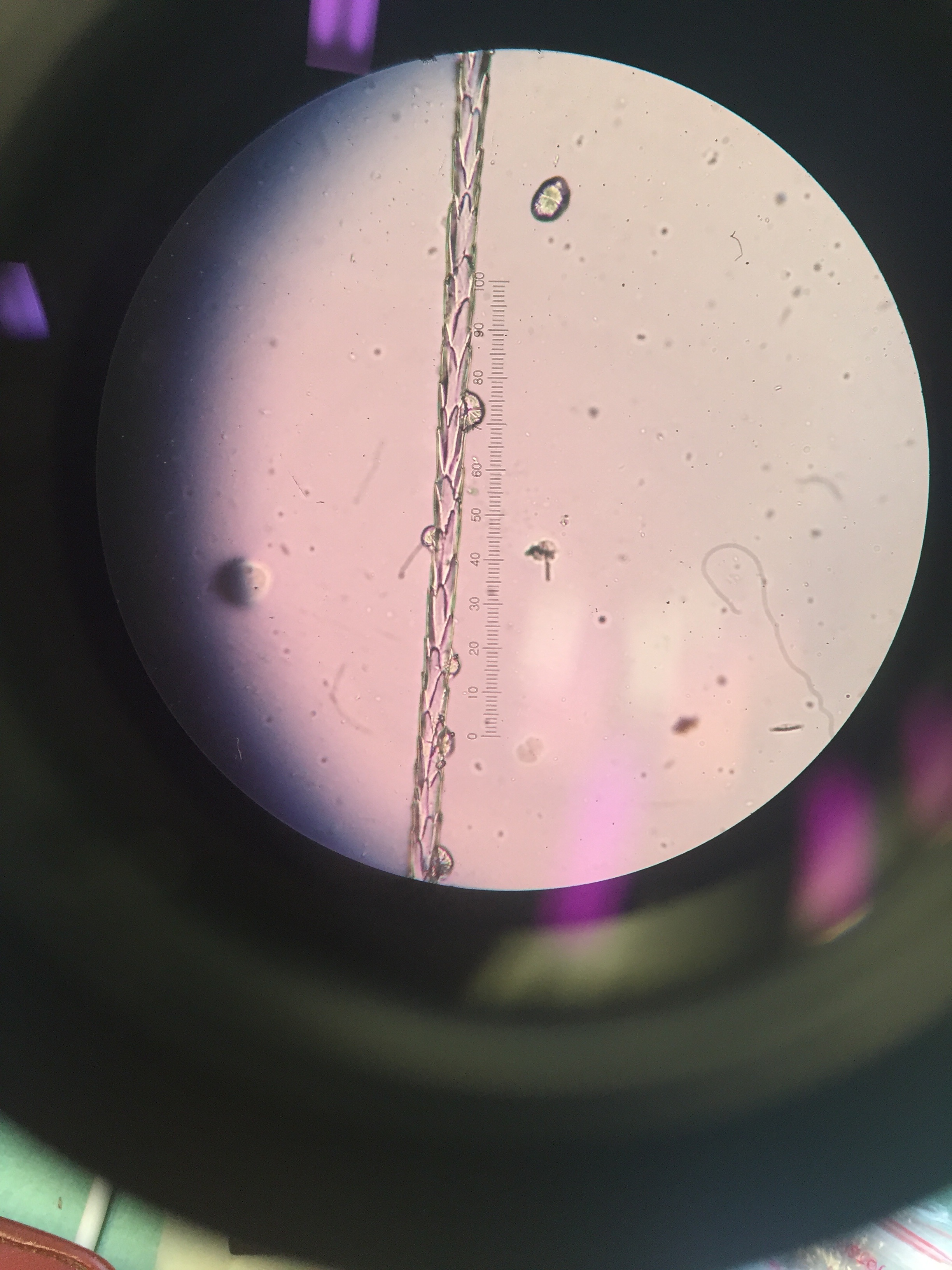
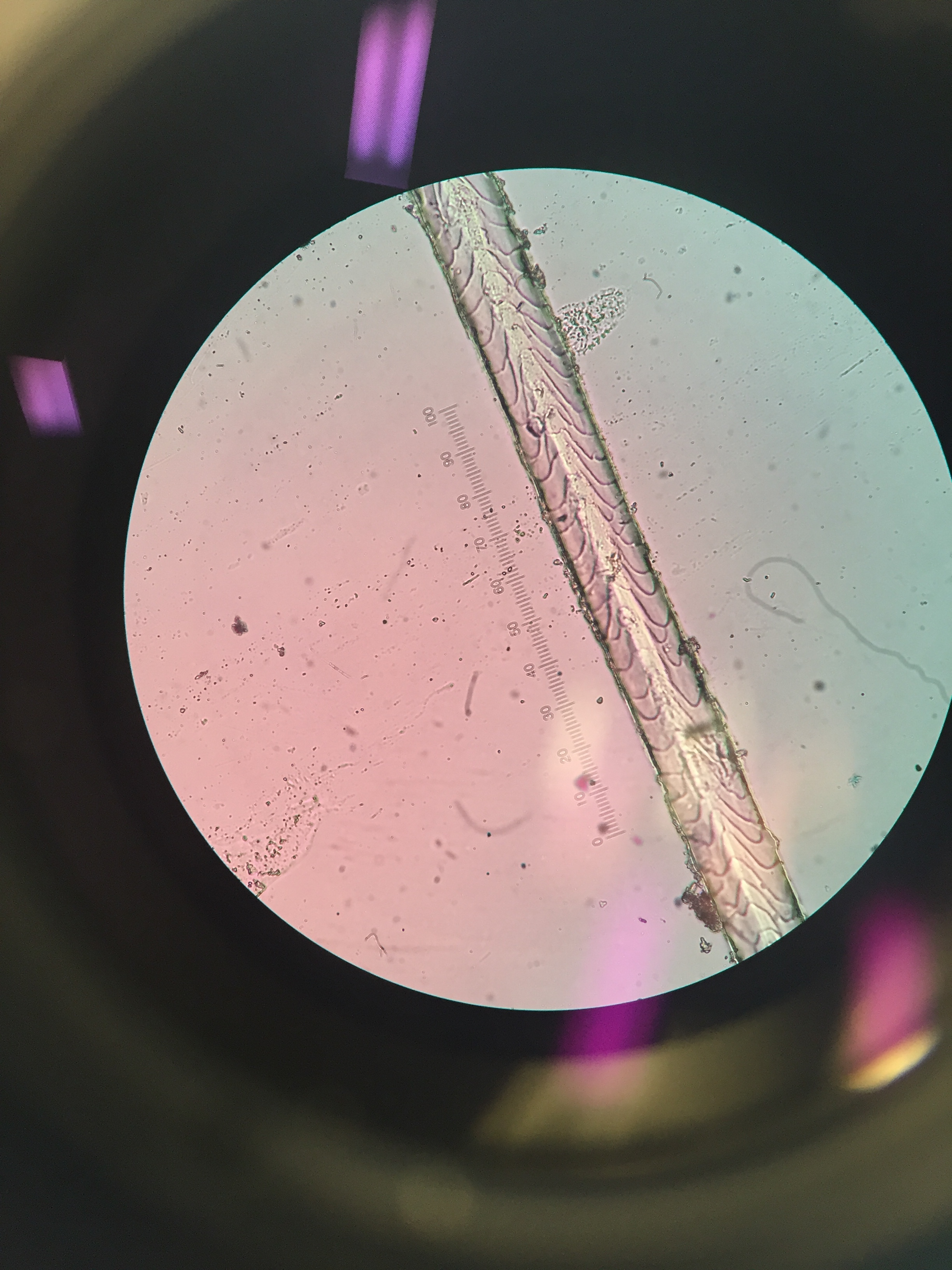
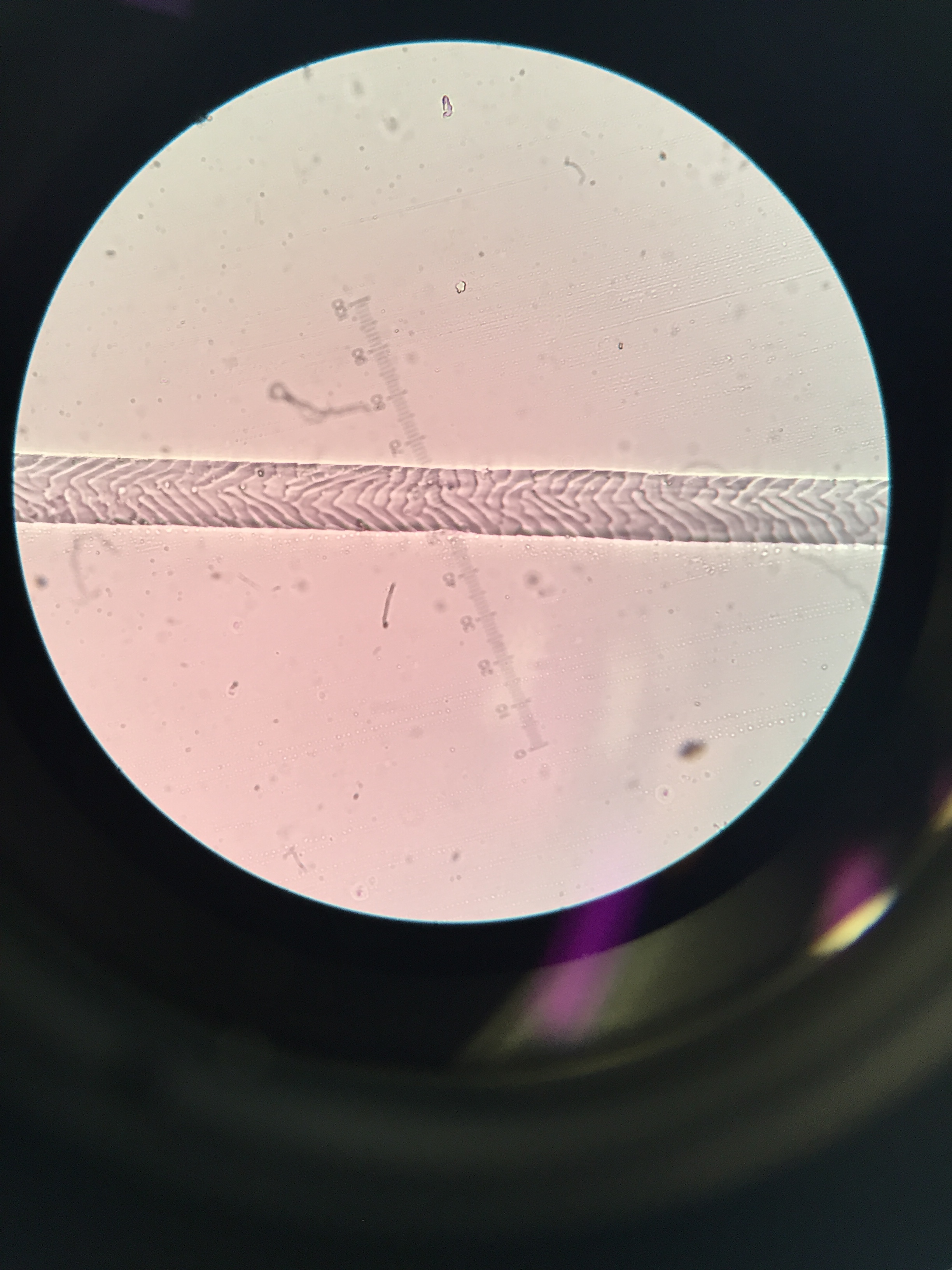
Surprisingly (to me) different species actually have pretty different scale patterns under magnification. I made some reference slides for myself of the species unlikely to be in the keys and guides I used, like grey squirrel and domestic dog. I took a few quick photos through the microscope with my phone:
One of the guides I used was written by Hillary Keogh in 1985 (fetchingly entitled A photographic reference system based on the cuticular scale patterns and groove of the hair of 44 species of southern African Cricetidae and Muridae), and I fortuitously managed to get hold of her original slides some time ago. She describes southern vlei rat (Otomys irroratus) hair as “Covered with lanceolate-pectinate scales. Almost petal at start of base… pattern gradually flattens out to become an irregular waved mosaic pattern.” Accompanying this helpful text was a terrible quality black and white photo of something I assumed was meant to be the hair. After struggling for some time, I suddenly realised I actually had in my possession the very slide the hair I was squinting at was on. From there on, it got easier. Contrary to that photo, Otomys hair is very distinctive, so I could move through a bunch of the samples I wanted to check quite quickly.
For the most part, my guesses based on macroscopic structure of the hair were correct. But I’m glad I could confirm them; I made a few corrections and sometimes found more than one type of hair present. In total I checked 84 samples which means I’m now finished! I must admit I vastly underestimated how long it would take to get here. Now the real fun (read: panic) begins… data cleaning and analysis in preparation for SAWMA 2017 next week!